International Journal of Biology and Nanobiomaterials1 (2021) 25-36
A review on SARS-CoV-2
DhivyaSekara, PriyaMurugananthama, SudarkodiVenkadesana, VigneshPillaia*
aDepartment of Microbiology, PSG College of Arts & Science, Coimbatore
ARTICLE INFO
Article history:
Received 15 Jan 2021
Revised 19 Feb 2021
Accepted 01 Mar 2021
Available online 05 Mar 2021
Keywords:
SARS-CoV-2
COVID-19
Cardiovascular diseaseCoronaviridae
Hydroxychloroquine
ABSTRACT
The novel corona virus SARS-CoV-2 causes severe acute respiratory syndrome along with other complexities that has led to the rise of COVID-19 pandemic. Its severe clinical manifestations, confirmed cases and mortality rate have increased vigorously. Phylogenetic studies reveal that SARS-CoV-2 is closely associated with the Chiropterasp, but this concept is still under discussion which renders the origin of the SARS-CoV-2 not recognized. SARS-CoV-2 can be transmitted from human to human and human to animal transmission is also reported. The mode of infection in a gist can be explained as when the spike (S) protein of the virus binds to Angiotensin-Converting Enzyme 2 (ACE 2) receptor of the host cell and enters into the susceptible cell to initiate replication. Its clinical manifestations have been reported as loss of smell or taste, breathlessness, and multiple organ dysfunctions, especially for individuals with co-morbidities. This review discussed the structure composition of SARS-CoV-2, route of spread, types of susceptible patients, mode of replication of the virus, clinical manifestations, pathogenesis, lab diagnostic methods, recent trends in treatment and prevention.
- Introduction
Coronaviridae is a family of spherical, enveloped, large virus containing a single stranded RNA as the genome. The family is named as ‘corona’ for the crown like morphology seen under electron microscopy (Willey et al., 2011). These are relatively large RNA viruses, diameter ranging from 118- 136 nm. It possesses a genome size of 25-32 kb enclosed within an envelope bearing large peplomer spikes which protrude from the envelope. Coronaviruses belong to the subfamily Coronavirinae in the family Coronaviridae of the order Nidovirales(Payne et al., 2017). Coronavirinae are found extensively in mammals that commonly causes transmissible cold to more complications.Fig.1 showed that subfamily Coronavirinae further contains 4 genera that have been classified below including the strain infecting humans. SARS-CoV-2 falls under the category betacoronavirus.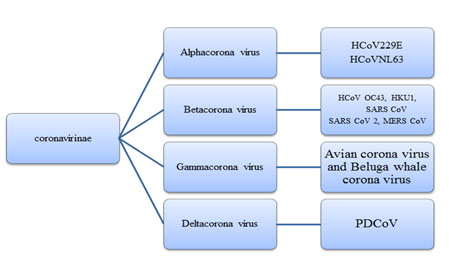
Fig. 1 Classification of Coronavirinae
COVID-19 is a highly transmittable pulmonary disease caused SARS-CoV-2. In the name COVID-19 “CO” stands for corona, “VI” stands for virus, “D” stands for disease and 19 stands for year 2019 (UNICEF/UNI220408/PACIFIC). Bats and pangolins are considered most likely as the natural host for SARS-CoV-2. RmYN02 virus from Rhinolophusmalayanus(Malayan horseshoe bat) is more closely related to SARS-CoV-2 than to pangolin CoV and that pangolin could not be the only intermediate host (Zhou et al., 2020; Liu et al., 2020). At complete viral genome analysis RmYN02 shares 93.3% nucleotide identity with SARS-CoV-2.
Taxonomy
Realm : Riboviria
Phylum : Incertaesedis
Order : Nidovirales
Suborder : Cornidovirineae
Family : Coronaviridae
Subfamily : Orthocoronavirinae
Genus : Betacorona virus
Subgenus : Sarbecovirus
Species : Severe Acute Respiratory Syndrome (SARS)
Strain : SARS Corona virus 2/ COVID 19 virus/ 2019 nCoV (Gorbalenya et al., 2020).
- Structure and composition
SARS-CoV-2 is a large virus with a diameter (60-140) nm (Cascella et al., 2020). These are spherical and enveloped virus. The envelope is made up of a phospholipid bilayer derived from the hos cell system. The viral envelope containing of envelope (E) proteins, bromodomain (BRD2) proteins, membrane (M) proteins, hemagglutinin esterase (HE) dimers and spike (S) glycoproteins which protrudes from the envelope. S proteins consist of ectodomain 160 Å long trimer (Bianchi et al., 2020; Meara et al., 2020; Klausegger et al 1999; Walls et al., 2020). The envelope has contained viral nucleocapsid (N) protein surrounds the RNA genome. SARS-CoV-2 has a positive single stranded RNA as its genetic material. It is found to have 8903 adenosines, 5482 cytosines, 5852 guanines, and 9574 thymine units that add up to 29811 nucleotides in total (Sah et al., 2020). It has the genome size of 29.8 Kb.The genome of SARS-CoV-2 has 14 ORF regions encoding 27 proteins (Wu et al., 2020) ORF1ab is the longest ORF occupying 2/3 of the entire sequence (Fehr and Perlman 2015) and it codes for a replicasepolyprotein 1ab which contains 7096 amino acids. The replicase protein is cleaved by 2 proteases. Multifunction related to transcription and replication of RNA (Wu et al., 2020) has been in regard to this polyprotein. PP1ab and PP1a proteins are encoded by ORF1ab and ORF1a genes at the 5’ end. PP1ab has Nsp1 to 10, 12, 13, 14 and 16 units. The PP1a also has Nsp1 to10 units. The 3’ end has 4 structural protein such as S, E, M and N protein along with 8 accessory proteins- 3a, 3b (22 amino acid), p6, 7a, 7b, 8b (121 amino acid), 9b and ORF 14.(Wu et al., 2020). ORF2-10 encodes S, M, N, E proteins which helps for structural organization and other accessory proteins. S, M, E proteins help in viral coat production. N protein helps in RNA packaging in virion (Wu et al., 2020). Structure of SARS-Co-2 showed in Fig.2.
2.1 Epidemiology of SARS-CoV-2
The new strain was first officially reported at Wuhan, China during the month of December 2019 and then it was widely spread throughout the world denoted as pandemic (WHO who.int/news-room/detail/27-04-2020). From china it has spread across all major continents including America South-Asia, Russia, etc.,(WHO 20200420-sitrep-91-COVID-19)
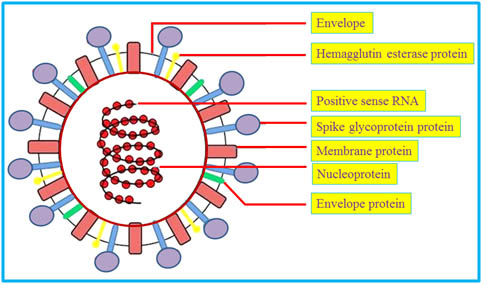
Fig.2 Structure of SARS-CoV-2
2.2 Mode of outspread
COVID-19 virus spreads through to the droplets (Alford et al., 1966). Droplets (5 µm or more in diameter) and droplet nuclei (1- 4µm in diameter that results from the evaporation of larger droplets) have expelled by the infected individual while coughing, vocalization and sneezing (Xie et al., 2009; Willey et al., 2011). Each droplet (10µm in diameter) moves about 100m/sec or more than 200Mi/hrs. Viral loaded, mucus is also responsible for the transmission. Ocular secretion from the infected patient has confirmed the presence of virus particles (Colavita et al., 2020). Direct contact transmission includes touching, kissing, copulation, via oral secretions or body lesions(Willey et al., 2011). Close contact and fomites of an infected person or surface contact with contaminated environment can lead to the transmission of COVID-19 (Dick et al., 1987; Moser et al., 1979) transmission through fomites such as drinking vessels, bedding, is also possible, etc., No evidence has been found to reportvertical transmission of the virus. However,during breast feeding droplet from an infected mother can transmit SARS-CoV-2 to the neonate (Karimi-Zarchi et al.,2020).Reports claim pregnant women to be more prone to COVID-19 (Chen et al., 2020). According to Zhang et al.,2020, SARS-CoV-2 was found in fecal specimen during diagnosis, therefore improper sewage disposal can also spread the virus. Indirect transmission of SARS-CoV-2 has also been witnessed by asymptomatic infected persons (Cai et al., 2020).‘Super-spreader’ events may be responsible for 80% of COVID infections (Voanews.com/covid-19-pandemic, may 23,2020) super-spreaders are the potential high-risk factors which can infect large number of populations coming to contact simultaneously (Timesofindia.indiatimes.com, may 16,2020).
2.3 Susceptibility
Risk factors for COVID-19 includes co-occurrence of hypertension, lung diseases, and heart disease (Yang et al., 2020) SARS-CoV-2 are found to infect more of the male population when compared to females (Badawi and Ryoo, 2016; Channappanavar et al., 2017). Senior citizens and people with co-morbidities are at a higher risk of acquiring SARS-CoV-2 (Zhang et al., 2020a). Pregnant women, diabetic patients, blood group A are more likely to be infected with SARS-CoV-2 compared with other non-A blood groups (Chen et al., 2020; Iacobellis et al., 2020, Zhao et al., 2020). Tobacco smoking increases ACE 2 which is the entry point for the virus and increases the risk for viral binding and entry (Cai et al., 2020). Cats can be infected with SARS-CoV-2 and can be spread to other cats.Four tigers and three lionswere found positive for SARS-CoV-2 at the Bronx zoo, a Pomeranian, a German shepherd at Hongkong, and a domestic cat at Belgium was also reported positive for SARS-CoV-2 (Mallapaty, 2020). Human can spread the virus to animals whereas no clear evidence is provided to prove that animals can spread SARS-CoV-2 the other way around (Nationalgeographic.com aprl 22,2020).
2.4 Replication
The replication of any virus involves 7 important stages- attachment, entry, replicase protein expression, replication, transcription, assembly and release. SARS-CoV-2 uses ACE 2 receptor for entry and serine protease TMPRSS 2 for S protein priming (Hoffmann et al., 2020). Spike glycoprotein receptor binding domain (RBD) found in the envelope of the virus attaches to Angiotensin converting enzyme 2 (ACE 2) receptor of the host cell by this the virus is taken into the cell through a process called pinocytosis (Wan et al., 2020). It enters the cell as an endosome. Upon entry into the host cell the viral RNA gets released into the cytoplasm. ORF 1a and ORF 1ab are translated to produce PP1a and PP1ab which are cleaved by proteases that are produced by ORF1a to give 16 Nsps. RNA replicase transcriptase complexes, is produced by the 16 Nsps. These complexes gather at the perinuclear region inside the endoplasmic reticulum to produce the negative sense RNA via replication and transcription. During the process of replication, the first full length negative strand is obtained. This negative strand RNA is used as a template to produce numerous positive sense RNA strands. Through discontinuous transcription all the necessary structural proteins get synthesized. In this process sub genomic negative sense RNA is also produced by joining varying length of 3’ end of genome with 5’ leader sequence required for translation. The sub genomic positive sense mRNA, are reproduced through the transcription of sub genomic negative strand RNA. The first ORF which is closest to the 5’ end is the only ORF that gets translated. At the endoplasmic reticulum-Golgi intermediate compartment, the structural proteins are assembled within the nucleocapsid and envelope of the virus. In this way complete virions are released from the infected cells (De et al., 2016).
2.5. Clinical manifestation
Symptom of COVID-19 appear in about 2-14 days which includes fever, cough, fatigue, pneumonia and finally a cytokine storm which can lead to ARDS and kidney failure. In some patients the symptoms reported include sore throat, aches and pain, diarrhea and nausea, headache or hemoptysis. Alveolar damage has also been seen in infected elderly men. Conjunctivitis, loss of taste or smell, skin rashes, difficulty in breathing, chest pain, sub-acute thyroiditis, loss of speech or movement, fever with upper respiratory tract infection with lymphopenia or leukopenia, and even septic shock. If untreated it leads to organ dysfunction and death. Some patients are asymptomatic (WHO interim guidance document;who.int/docs/default-source, Brancatella et al., 2020; Zu et al 2020).
2.5.1 Pathogenesis
Pathological changes in infected individual are classified into 3 stages. They are asymptomatic state, upper airway and conducting airway response and hypoxia, ground glass infiltrates and progression to ARDS. The epithelial cells present in the nasal cavity, apical cilia on airway cells and microvilli on type 2 cells are binding site and assist the entry for SARS-CoV-2 virus. It binds to the cells and starts its replication. During this stage multiplication of virus occurs by restricted innate immune response. The second stage is manifested within the next few days. The virus gets replicated and spread throughout the respiratory tract. In this stage increased and strong innate immune response, increased nasal secretion and mucus is observed. Due to beta and lambda interferon response from infected epithelial cell leukocytes, monocytes, activated neutrophils, eosinophils, endothelial cells, fibroblast cells- secretion of CXCL10 inflammatory chemokine have been studied (Mason, 2020; Liu et al., 2011). At this stage the virulence of the disease will be mild and mostly limited to upper respiratory tract along the conducting airways. At third stage, the virus migrates to the alveoli and infects alveolar type 2 cells. The viral particle multiples within type 2 cells and virions are released by lysing the cell with self- replicating pulmonary toxin and infecting the adjacent units. Secondary pathway for epithelial regeneration will be activated. Diffuse alveolar damage (DAD) with hyaline membrane (membrane with proteins, dead cells, surfactant, difficulty in breathing and exchange of gases) and multinucleated giant cells (MGC), severe scaring and fibrosis are the severe changes forming hypoxia, ground glass infiltrates and progression to ARDS.
2.5.2 Lab diagnosis
Sputum, bronchial specimen, bronchoalveolar lavage fluid, nasal, nasopharyngeal swabs, oropharyngeal swabs, saliva, tracheal samples are the specimens used to test COVID-19 (Wan et al., 2020; cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html). According to the report, 7.11x 108 RNA copies was found in a throat swab on the 4th day of infection (Wolfel et al., 2020). He also emphasized that enormous amount of pharyngeal viral discharge was observed during first week of symptoms. CXCL 10 activation was also used as a diagnostic biomarker in SARS (Tang et al., 2005; Mason, 2020). RT PCR test is widely used for the qualitative detection of viral RNA from COVID-19 infected patients. It helps to quantify the viral load, stage of infection, progression of infection and the treatment required (LabCorpcovid 19 RT-PCR test EUA summary 2020). CT chest computed tomography scan and magnetic resonance imaging MRI scan can be useful in finding out COVID-19 with their co rad values. Serological antibodies for COVID-19 get developed during the 2nd week of infection using those antibodies, quantification of antibodies and diagnosis of COVID-19 can be made (Wölfel et al 2020). CRISPR based DETECTOR assay helps to detect the virus faster which has become the alternative for RT PCR assay (Broughton et al., 2020). IgM antibodies are also used for the detection of COVID-19. EUA-FDA approves SARS-CoV-2 antibody test that can reveal the results in 15 min (NBC news, 9 may 2020). EUA- FDA approves new Rutgers saliva test for SARS-CoV-2 (Rutgers.edu, 13 April 2020). According to Chen et al., 2020the rapid kit can diagnose COVID-19 in 10 min which contains lanthanide doped polystyrene nanoparticles used in a lateral flow immunoassay for the detection of anti SARS-CoV-2 IG2 in human serum.
- Treatment
Currently no confirmed treatment has been developed for COVID-19. Vaccine development has been achieved with considerable curative activities. Until then, supportive treatments for symptoms are recommended. High flow nasal oxygen therapy can be provided, the patients requiring endotracheal intubation use of low tidal volume with 6mL/kg per predicted body weight with a plateau airway pressure <30 cm H2O can be maintained if possible, positive end expiratory pressure can be increased from moderate to high if needed, administration of neuromuscular blockade for ventilator desynchrony, increased airway pressure and hypoxemia. Delivering a recruitment maneuver and high PEEP improves oxygenation and reduce need for other rescue therapy. Placing prone positioning helps to improve oxygenation for worsening hypoxemia, PaO2 FiO2 <100-150 mm Hg. Usage of conservative fluid management in patients with SARI when there is no evidence of shock. Renal placement therapy is for oliguric renal failure, acid base management, and negative fluid balance. Inhalation of nitic oxide can be 5-20 ppm may improve oxygenation (Matthay et al., 2020). Rescue therapy with high dosage of vitamin C is recommended (Truwit et al., 2019).
Remdesivir acted against SARS-CoV-2 and significant result was achieved (Holshue et al., 2020). Gautret et al., reported that hydroxychloroquine and azithromycin treatment found to be efficient against the virus and significantly reduced the viral load. But usage of hydroxychloroquine for COVID-19 treatment is temporarily suspended in the solitary trial by world health organization (Gautret et al., 2020). In India revised advisory issued by ICMR has recommended use of hydroxychloroquine as prophylaxis (Economic times 26th may 2020). Ivermectin reduced the viral RNA 99.8% after 48 hrs (Caly et al., 2020). Abidol, lopinavir/ritonavir, chloroquine, alpha interferon, nucleoside analogues, neuraminidase inhibitors, peptide EK1, anti-inflammatory drugs, RNA synthesis inhibitor TDF, 3TC can also be used for SARS-CoV-2. ShuFengJie Du capsules, Lianhuaquingwen capsule, shuanghuanglian oral liquid belongs Chinese traditional medicine could be used against SARS-CoV-2 (Lu, 2020; Ni et al., 2020) CAP 1002 contains allogenic cardio sphere derived cells (cdc) treatment found to be effective in critically ill COVID-19 patients (Singh et al.., 2020). The drugs such as favipiravir, darunavir/cobicistat, camoststmesilate/ nafamostat, tocilizumab, colchicine, baricitinib, aviptadil, eculizumab, meplazumab, novaferon, niclosamide, ciclesonide a monoclonal antibody are used for the symptoms and treatment of COVID-19 are currently used or under clinical trials list (Scavone et al 2020; Zheng et al., 2020; Jeon et al., 2020). Sofosbuvir, galidesivir and tenofovir showed good result against SARS-CoV-2 (Elfiky, 2020) Auranofin found to reduce virus 95% in 48hrs after infection (Rothan et al., 2020) Synthetic recombinant interferon alpha (Lu et al 2020), immunoglobulin (Jawhara, 2020), thymosin alpha 1(Liu et al 2020), plasma therapy provided the promising result for the treatment of COVID-19 (Chen et al., 2020).
3.1 Prevention and control
Maintain hand hygiene, follow social distancing by maintaining at least 1 m distance from others, avoid touching contaminated surfaces. Public often using surfaces like door knobs, computer mouse, etc., minimize the skin exposure, avoid touching eyes, nose and mouth. Use mask when going outdoor. Use tissue paper or cover mouth and nose while coughing or sneezing. Self-isolation and proper medication through doctors is must, when encountered with any of the symptoms (who.int/emergencies/diseases/novel-coronavirus-2019). People have to access clean water, decent sanitation, basic health care infrastructure, avoid travel by air to other countries, avoid uncooked food, smoking, unpasteurized dairy products, avoid skin perforating procedures like acupuncture, body piercing, tattooing, venipuncture, sharing of razors. Avoid pet animals like cat, civet, dogs, and feline domestic as well as wild animals. Avoid swimming in public pools or non-chlorinated public water (Willey et al., 2011). Wash hands with soap, alcohol based sanitizer, usage of personal protective equipment (PPE), In case of health care workers eye protection glasses must also be provided, personal hygiene should be maintained, avoid skin perforating injury, disinfecting the equipment handled by workers in COVID-19 area and often clean the environment. Mask such as N95, FFP2, FFP3, PPE, gloves, long sleeved gowns are compulsory. Adequately ventilated rooms are used to perform aerosol generating procedures 40(whointerimguidancedocument). To control the spread the infected person should be identified rapidly then isolated and proper medication must be provided. Awareness must be provided to the public and health care workers (Xiao and Torok, 2020).
- Conclusion
Due to the emergence of COVID-19, all the countries are facing tremendous loss in all aspects. Many countries are struggling for life of people, virus control, self-sufficient food materials, medicines, etc., since COVID-19 is highly contagious, people cooperation, self-isolation after air travel, curfew, social distancing, wearing PPE found to be effective measure. Apart from this development and maintenance of public health care centers with experts, advanced lab equipment, research centers, implementation of government policies for the transmission and prevention of disease, investing money in genome analysis, development of advanced diagnosis kit and equipment, microbiological testing laboratories in more numbers has to be encouraged. Due to the mutation occurring in virus, emergence of new strains are possible so genetic studies are important for drug discovery and drug delivery. Creating awareness, educate, train the public regarding the epidemiology of the infection and personal health hygiene is mandatory. To control measures can be done through removing the source of infection from the public, breaking the chain between the source and public, raising herd immunity. The production of vaccine and vaccination will be the prevention measure for future generation.
Funding: The authors received no specific funding for this work.
Conflicts of Interest: None
References
- Alford, R. H., Kasel, J. A., Gerone, P. J., & Knight, V. 1966. Human influenza resulting from aerosol inhalation. Proceedings of the Society for Experimental Biology and Medicine, 122, 800-804.
- Badawi, A., &Ryoo, S. G., 2016. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. International Int J Infect Dis, 49, 129-133.
- Bianchi, M., Benvenuto, D., Giovanetti, M., Angeletti, S., Ciccozzi, M., &Pascarella, S., 2020. Sars CoV2 Envelope and membrane proteins: structural differences linked to virus characteristics? BioMed Research International. https://doi.org/10.1155/2020/4389089.
- Brancatella, A., Ricci, D., Viola, N., Sgrò, D., Santini, F., &Latrofa, F., 2020. Subacute thyroiditis after SARS-CoV-2 infection. J ClinEndocrinolMetab, 105, 276.
- Broughton, J. P., Deng, X., Yu, G., Fasching, C. L., Servellita, V., Singh, J., … & Zorn, K. (2020). CRISPR–Cas12-based detection of SARS-CoV-2. Nature Biotechnology, 1-5.
- Cai, G., Bossé, Y., Xiao, F., Kheradmand, F., & Amos, C. I., 2020. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. American journal of respiratory and critical care medicine, (ja).
- Cai, J., Sun, W., Huang, J., Gamber, M., Wu, J., & He, G., 2020. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg Infect Dis, 26, 1343-1345.
- Caly, L., Druce, J. D., Catton, M. G., Jans, D. A., &Wagstaff, K. M., 2020. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res, 104787.
- Cascella, M., Rajnik, M., Cuomo, A., Dulebohn, S. C., & Di Napoli, R., 2020. Features, evaluation and treatment coronavirus (COVID-19). Statpearls [internet].
- Channappanavar, R., Fett, C., Mack, M., Ten Eyck, P. P., Meyerholz, D. K., & Perlman, S., 2017. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol, 198, 4046-4053
- Chen, H., Guo, J., Wang, C., Luo, F., Yu, X., Zhang, W., & Liao, J., 2020. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet, 395, 809-815.
- Chen, L., Xiong, J., Bao, L., & Shi, Y., 2020. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis, 20(4), 398-400.
- Colavita, F., Lapa, D., Carletti, F., Lalle, E., Bordi, L., Marsella, P., &Ippolito, G., 2020. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 17, M20-1176.
- De Wit, E., van Doremalen, N., Falzarano, D., & Munster, V. J., 2016. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol, 14(8), 523.
- Dick, E. C., Jennings, L. C., Mink, K. A., Wartgow, C. D., & Inborn, S. L., 1987. Aerosol transmission of rhinovirus colds. J Infect Dis. 156, 442-448.
- Elfiky, A. A. (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci, 15, 253:117592.
- Fehr, A. R., & Perlman, S., 2015. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 1282, 1-23..
- Gautret, P., Lagier, J. C., Parola, P., Meddeb, L., Mailhe, M., Doudier, B., &Honoré, S., 2020. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents, 105949.
- Gorbalenya, A. E., Baker, S. C., Baric, R. S., de Groot, R. J., Drosten, C., Gulyaeva, A. A., &Penzar, D., 2020. The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5: 536–544.
- Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., & Müller, M. A., 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181, 271-280.e8
- Holshue, M. L., DeBolt, C., Lindquist, S., Lofy, K. H., Wiesman, J., Bruce, H., & Diaz, G. 2020. First case of 2019 novel coronavirus in the United States. N Engl J Med, 382, 929-936.
- https://economictimes.indiatimes.com/news/politics-and-nation/no-major-side-effects-of- hydroxychloroquine-should-be-continued-as-preventive-treatment-for-covid-19-icmr/videoshow/76007773.cms
- https://genetics.rutgers.edu/news-events/news/513-4-13-2020-new-rutgers-saliva-test-forcoronavirus-gets-fda-approval https://timesofindia.indiatimes.com/city/ahmedabad/ahmedabad-700-super-spreaders-found-coronavirus-positive-in-a-week/articleshow/75776329.cms
- https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19.Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19).
- https://www.nationalgeographic.com/animals/2020/04/tiger-coronavirus-covid19-positive-test-bronx-zoo/
- https://www.nbcnews.com/news/us-news/fda-grants-emergency-approval-15-minute-coronavirus-test-n1171131
- https://www.voanews.com/covid-19-pandemic/superspreader-events-may-be-responsible-80-covid-infections
- https://www.who.int/docs/default-source/inaugural-who-partners-forum/coronavirus-poster-english-srilanka.pdf?sfvrsn=289dedc3_0
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public.
- https://www.who.int/news-room/detail/27-04-2020-who-timeline—covid-19
- Iacobellis, G., Penaherrera, C. A., Bermudez, L. E., &Mizrachi, E. B., 2020. Admission Hyperglycemia and Radiological findings of SARS-COv2 in patients with and without Diabetes. Diabetes Res ClinPract, 164, 108185.
- Jawhara, S. 2020. Could Intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients?. Int. J. Mol. Sci., 21, 2272.
- Jeon, S., Ko, M., Lee, J., Choi, I., Byun, S. Y., Park, S., & Kim, S., 2020. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother, 64, e00819-20.
- Karimi-Zarchi, M., Neamatzadeh, H., Dastgheib, S. A., Abbasi, H., Mirjalili, S. R., Behforouz, A., &Bahrami, R., 2020. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal PediatrPathol, 39, 246-250.
- Klausegger, A., Strobl, B., Regl, G., Kaser, A., Luytjes, W., &Vlasak, R. 1999. Identification of a coronavirus hemagglutinin-esterase with a substrate specificity different from those of influenza C virus and bovine coronavirus. J. Virol. 73, 3737-3743.
- LabCorpcovid 19 RT-PCR test EUA summary 2020 https://www.fda.gov/media/136151/download#:~:text=The%20COVID%2D19%20RT%2DPCR%20test%20is%20a%20real%2D,lower%20respiratory%20tract%20aspirates%2C%20bronchoalveolar
- Liu, M., Guo, S., & Stiles, J. K. (2011). The emerging role of CXCL10 in cancer. Oncol. Lett. 2, 583-589.
- Liu, P., Jiang, J. Z., Hua, Y., Wang, X., Hou, F., Wan, X. F., & Chen, J., 2020. Are pangolins the intermediate host of the 2019 novel coronavirus (2019-nCoV)?. PLOS Pathog. 16(5): e1008421.
- Liu, Y., Pang, Y., Hu, Z., Wu, M., Wang, C., Feng, Z., & Li, M., 2020. Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis, 16, 2150-2157.
- Lu, C. C., Chen, M. Y., & Chang, Y. L. (2020). Potential therapeutic agents against COVID-19: What we know so far. Journal of the Chinese Medical Association. 83, 534-536.
- Lu, H. 2020. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends, 14, 69-71.
- Mallapaty, S., 2020. Coronavirus can infect cats-dogs, not so much. Nature. doi: 10.1038/d41586-020-00984-8
- Mason, R. J. 2020. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 55: 200060
- Matthay, M. A., Aldrich, J. M., &Gotts, J. E., 2020. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med, 8, 433-434.
- Meara, M. J., Guo, J. Z., Swaney, D. L., Tummino, T. A., &Hüttenhain, R. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing
- Moser, M. R., Bender, T. R., Margolis, H. S., Noble, G. R., Kendal, A. P., & Ritter, D. G., 1979. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol, 110, 1-6.
- Ni, L., Zhou, L., Zhou, M., Zhao, J., & Wang, D. W. 2020. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front Med, 14, 210-214.
- Payne, S., 2017. Family Coronaviridae. Viruses, 149-158.
- Rothan, H. A., Stone, S., Natekar, J., Kumari, P., Arora, K., & Kumar, M., 2020. The FDA-approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology. 547,7-11.
- Sah, R., Rodriguez-Morales, A. J., Jha, R., Chu, D. K., Gu, H., Peiris, M., &Zambrano, L. I., 2020. Complete genome sequence of a 2019 novel coronavirus (SARS-CoV-2) strain isolated in Nepal. MicrobiolResourAnnounc, 11, e00169-20.
- Scavone, C., Brusco, S., Bertini, M., Sportiello, L., Rafaniello, C., Zoccoli, A., & Capuano, A. 2020. Current pharmacological treatments for COVID‐19: what’s next?. J. Pharmacol.. 177, 4813-4824.
- Singh, S., Chakravarty, T., Chen, P., Akhmerov, A., Falk, J., Friedman, O., &Marbán, E. 2020. Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res. Cardiol. 115:36.
- Tang, N. L. S., Chan, P. K. S., Wong, C. K., To, K. F., Wu, A. K. L., Sung, Y. M., & Lam, C. W. K. 2005. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clinical chemistry, 51(12), 2333-2340.
- Truwit, J. D., Hite, R. D., Morris, P. E., DeWilde, C., Priday, A., Fisher, B., &Nanchal, R. 2019. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. Jama, 322, 1261-1270.
- Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., &Veesler, D., 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 181, 281-292.e6.
- Wan, Y., Shang, J., Graham, R., Baric, R. S., & Li, F., 2020. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol, 94(7).
- Wan, Y., Shang, J., Graham, R., Baric, R. S., & Li, F., 2020. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol, 94(7).
- WHO 20200420-sitrep-91-COVID 19https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200420-sitrep-91-covid-19.pdf?sfvrsn=fcf0670b_4
- Willey, J. M., Sherwood, L., &Woolverton, C. J., 2011. Prescott’s microbiology. Eighth edition NY: McGraw-Hill Higher Education.
- Wölfel, R., Corman, V. M., Guggemos, W., Seilmaier, M., Zange, S., Müller, M. A., &Hoelscher, M., 2020. Virological assessment of hospitalized patients with COVID-2019. Nature, 581, 465-469.
- World Health Organization. 2013. Interim guidance document. Clinical management of severe acute respiratory infections when novel coronavirus is suspected: What to do and what not to do.
- Wu, A., Peng, Y., Huang, B., Ding, X., Wang, X., Niu, P., & Sheng, J., 2020. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 27, 325-328.
- Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., … &Zheng, M., 2020. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 10, 766-788.
- Xiao, Y., &Torok, M. E. 2020. Taking the right measures to control COVID-19. Lancet Infect Dis 20, 523-524.
- Xie, X., Li, Y., Sun, H., & Liu, L., 2009. Exhaled droplets due to talking and coughing. J R Soc Interface. 6, S703-14.
- Yang, J., Zheng, Y., Gou, X., Pu, K., Chen, Z., Guo, Q., & Zhou, Y., 2020. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis, 94, 91-95.
- Zhang, J. J., Dong, X., Cao, Y. Y., Yuan, Y. D., Yang, Y. B., Yan, Y. Q., &Gao, Y. D., 2020. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 75, 1730-1741
- Zhang, J., Wang, S., &Xue, Y., 2020. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. Journal of medical virology, 92(6), 680-682
- Zhao, J., Yang, Y., Huang, H. P., Li, D., Gu, D. F., Lu, X. F., & He, Y. J., 2020. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. medRxiv.https://doi.org/10.1101/2020.03.11.20031096.
- Zheng, F., Zhou, Y., Zhou, Z., Ye, F., Huang, B., Huang, Y., &Niu, P., 2020. A Novel Protein Drug, Novaferon, as the Potential Antiviral Drug for COVID-19. medRxiv. https://doi.org/10.1101/2020.04.24.20077735.
- Zhou, H., Chen, X., Hu, T., Li, J., Song, H., Liu, Y & Hughes, A. C., 2020. A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr Biol. 11, 2196-2203.e3.
Zu, Z. Y., Jiang, M. D., Xu, P. P., Chen, W., Ni, Q. Q., Lu, G. M., & Zhang, L. J., 2020. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology, 200490.
