International Journal of Biology and Nanobiomaterials1(01), 2021, pp.1-12
Review
Bio-polymer of chitosan based nanocomposites for biomedical applications
Srinivasan Subramanian*
Department of Environmental Science, Periyar University, Salem, Tamil Nadu, India
ARTICLE INFO
Article history:
Received 03 September 2021
Revised 05 October 2021
Accepted 08 October 2021
Available online 11 October 2021
Keywords:
Chitosan
Antibacterial activity
Drug delivery
Wound healing
Tissue engineering
ABSTRACT
Chitosan is natural biopolymer of amino polysaccharides which is derived from chitin. Chitosan has multifunctional biological properties such as non-toxic, biodegradability, biocompatibility, adsorption and antimicrobial properties. This important feature is significantly have been widely used in pharmaceutical and medical applications as synthetic material for bone tissue engineering, wound healing and drug delivery. Chitosan based nanocomposites have potential antimicrobial activity against different bacterial, viral and fungal pathogens it’s valuable for biological application of drug development system. Chitosan or combined with synthetic/ natural polymers based composites have been widely investigated for versatile biomedical use of drug delivery, wound healing, anti-microbial agent, tissue engineering, food preservative and anti-tumor activities. In this review will focus on the advantages of chitosan based nanocomposites or composites for pharmaceutical and biomedical applications.
1. Introduction
Recently, developed medicine combined with natural polymers in understanding molecular biology and cellular processes have been led to an increasing demand for medicinal field of biomedical applications. Those reasons have effectively stimulated researchers to research in the field of pharmaceutical and biomedical science, regarding chitosan. Natural biopolymer of chitosan is a linear polysaccharide prepared from N-acetylglucosamine of chitin. Which is known insoluble amino polysaccharide consist of β-(1,4)-2-
acetamino-2-deoxy-D-glucose unites. Chitosan have variant biological properties including biodegradability, biocompatibility, less-toxicity, low immunogenicity, antimicrobial activity, cost-effective and accessibility (Bozzuto et al., 2015; Johnston et al., 2007; He et al., 2019; Van et al., 2019). Chitosan is worldwide most abundant secondary biopolymer deacetylated form of chitin, which is predominantly naturally found in vertebrate animals, crab, lobster, insert and fungi and also occurring in the marine zooplankton species as well as jellyfish and coral (Bordi et al., 1991; Rha et al., 1984; Struszczyk et al., 1991; Abdou et al., 2008), chitin is also in wings like and lady bugs. In furthermore, yeast, plants and mushroom cell wall as well as animal cuticle (Nessa et al., 2010; Inmaculada et al., 2009). Structure and natural sources of chitosan is showed in Fig 1.
Naturally occurring polysaccharides such as cellulose, agarose, agar, pectin, dextran, carrageenan and alginic acid are acidic or neutral in nature. Cellulose and chitosan are naturally presenting polysaccharide, and their structural backbone of β-1,4-linked glucosamine along with N-acetylation its similar to cellulose. Although difference only in the repeated replacement of amino and hydroxyl group on C-2 position. But biological properties of cellulose difference from chitosan characteristics. Physicochemical properties of chitosan are slightly crystalline, hardness, tasteless and colorless material. Unique properties of chitosan may include polyoxy salt formation capability to form of metal ions chelate, film formation and soluble in different media, viscosity, solution formation and optical structural characteristics (Pillai et al., 2009). Chitosan is deacetylation form of chitin (1®4) linked 2-amino-2-deoxy- β-D-glucan. Chitin is highly insoluble and highly hydrophobic in water and organic solvent than compared to chitosan. But that is soluble in hexafluoroacetone, hexafluoroisopropanol, chloroalcohols combined with aqueous mineral acids solution and dimethylacetamide along with 5% of lithium chloride. Chitosan is soluble in diluted acids solution of glacial acetic acid, formic acid and N-methyl morpholine-N-oxide/H2O (Dutta et al., 2002). The chitosan structure is illustrated in (Fig.1).

Fig. 1. Structure of chitosan
Chitosan is broad range of commercial applications, but it has some limitation and drawbacks. In one of the well-known chitosan loaded drug delivery system it disturbs adsorption of fat soluble substances. Thus modification to change of pH value in stomach and it is lead to effects of pregnancy outcome and children growth. Therefore, chemically modified chitosan have been used and an impressed for many biomedical applications due to the presences of amino and two -OH functional groups, it’s chemically modified chitosan has enhancing biological properties and increasing solubility of solution.The chemical modification is mostly using of graft copolymerization (Jayakumar et al., 2005; Jenkins et al., 2001), cross-linking, etherification (Badawy et al., 2004;
Gorochovceva et al., 2004) and esterification (Yoshifuji et al., 2006).Chitosan sugar modification was first reported by Hall and Yalpani et al., in 1980. Recently, the World Health Organization has reported second most death of pathogenic microorganisms to cause infection disease of human health. Moreover, genetic mutated drug- resistance pathogenic microorganisms to an increased mortality in the worldwide. Therefore necessary most important to develop potential antimicrobial composite and it’s embedding multi-functionality as well as easily fabrication properties. Thus chemically modified chitosan or composite materials are known appropriatedcandidate for several biomedical applicationsshows in (Fig 2).
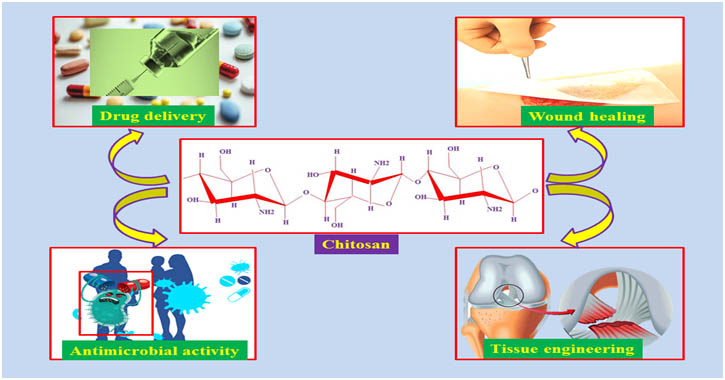
Fig 2. Biomedical Applications of chitosan
In this review paper is to highlight and discuss the recently developed various biomedical application of chitosan and chitosan emphasis or blended with composites materials.
- Biomedical applications of chitosan
2.1.Drug delivery
Recently, the drug delivery system is emerging and interesting subject especially using of chitosan based carrier materials for numerous applications of biological and pharmaceutical activities. Alternative method of disease treatment is well-known that chitosan based hydrogel materials, the active drug or substance is once loaded into a device or carrier materials it’s providing the prolong-time to release at a specific site and specific rate, reduced dosage of drug and thereby reducing side effects. The drug loaded chitosan based composites can be used to antibacterial, antifungal, anticancer, anti-inflammatory and gene delivery (Li et al., 2008; Badawy et al., 2014; Dong et al., 2018).
Jiang et al., to performed the drug release behavior against the antimicrobial activity, which is formulation of tetracycline hydrochloride loaded with chitosan/poly(latic acid) (Tet-CS/PLA) nanofiber membrane. The Tet-CS/PLA nanofiber composite have potential inhibitory activity of Staphylococcus aureus, it’s gradually increased inhibition activity to increasing of Tet concentration below 20%. The effective drug loaded chitosan based nanofiber has significant advantages of nontoxicity, solubility, pH-sensitivity, absorbability and furthermore, controlled biodegradability as well as sustained drug releases (Suwei et al., 2016). El-Alfy et al., have reported chitosan nanoparticles prepared and loaded by antibiotic of tetracycline, gentamycin and ciprofloxacin as drug delivery system for good improvement of antibacterial properties against both Gram positive and Gram negative bacteria. The antibiotic-chitosan nanoparticles loaded composite to fabricate with cotton/polyester had enhancement in the mechanical and physicochemical properties. Cong et al., have reported emodin drug encapsulated with alginate hydrogel/chitosan micelle composite, which results demonstrated that the biocompatible materials can be a promising sustained release or site-specific drug delivery system for instable or hydrophobic drugs. Most beneficial purpose of insulin drug delivery system for diabetic treatment, the thermosensitive of quaternized chitosan based hydrogel is prepared for insulin delivery system via the nasal cavity. This hydrogel form of drug delivery system results has demonstrated a desirable swelling rate, even porous structure and adequate encapsulation. These properties contribute to the prolonged release of insulin to improvement of therapeutic effects on diabetic treatments.
2.2. Wound healing
Skin is the largest organ of human musculoskeletal system, its outer cover of the human body and provided structure as well as fist protective barrier of the environmental factors. Wound healing is process of preventing deregulated homeostasis, epithelialization and inhibition of microbial contamination (Adeli et al., 2019). When the skin damaged, it immediately required ideal wound dressing materials its must be possess of exhibit properties to maintenances moister environment around in the wound area, permit gaseous exchange, adsorbed exudates from the wound secretion and protective against microorganisms. A good wound healing practices materials or scaffold should be possess important criteria’s such as non-toxic, non-adherent, non-allergenic, and biocompatibility with potential antimicrobial activity thus it’s to enhanced the wound healing process (Gomes et al., 2015; Khorasani et al., 208). Wound healing complication and delayed due to the microbial infection. Therefore, potential antimicrobial agents embedded wound healing dressing materials are great demand in the field of wound care market. The chitosan derivative can be used well-known promising dressing materials, thus high biodegradability, biocompatibility, non-toxicity, antimicrobial activity and good moisturizing capacity preserve around wound healing area. Furthermore, easily fabrication with desired materials and nanofibers resemble the ECM of the skin thus features further enhancing of wound healing process (Xu et al., 2018).
Sarhan et al., developed antimicrobial nanofibers for wound dressing by loading two natural plant aqueous extract. The fabricated nanofibers compositing honey, PVA and chitosan nanofibers (HPCS). Dried plant aqueous extract of Cleome droserifolia (CE) and Allium sativum are loaded within the HAPCS nanofibers, it’s noted as HPCS-CE, HPCS-AE and HAPCS-AE/CE nanofibers, respectively. Report revealed that the HAPCS-AE/CE nanofibers potential antibacterial activity against Methicillin resistant and S. aureus bacteria than compared to untreated control. Two plant aqueous extract combination with HPCS nanofibers potential enhancement of wound healing was observed in mice and, in further histological study was demonstrated to wound healing rate (Sarhan et al., 2016).
Carboxymethyl chitosan, hyaluronic acid and gelatin are cytocompatibility was examined by seeding of primary rabbit corneal epithelial cells (CEpCS) on the prepared scaffold to estimate the proliferation and growth rate. CEpCs/CMC scaffold was treated for the alkali-induced corneal injury in rabbits and wound healing process was evaluated that the CEpCs/CMC membrane could significantly improve corneal epithelial reconstruction and repair cornea transparency/thickness confirming this nanofiber membrane was effectively for corneal wound healing (Xu et al., 2018). Zhou et al., developed biocompatible nanofibrous scaffolds of photocrosslinkedmaleilated chitosan/methacrylatedpoly(vinyl alcohol) (MCS/MPVA), the nanofiber membrane water stability was improved by electrospining and photopolymerization method. The photocrosslinkednanofibrous cytotoxicity to evaluation by using of L929 cells that showed good proliferation and cellular compatibility it’s could be applied as potential wound dressing material (Zhou et al., 2017). Alavarse et al., prepared wound healing dressing scaffold which containing of PVA/chitosan and PVA/Chitosan/Tetracycline hydrochloride. This scaffold was investigated their physiochemical and biological properties of thermal, mechanical, morphological, antibacterial, cytotoxicity and drug release studies. Scaffold showed significant antibacterial activity against Gram positive and Gram negative bacteria of Staphylococcus epidermidis, Staphylococcus aureus and Escherichia coli. In-vitro MTT assay revealed good cytocompatibility of the drug loaded nanofiber scaffold, which indicated that scaffold could be used an antibacterial wound dressing material for tissue engineering application (Alavarse et al., 2017).
2.3. Antimicrobial activity
Chitosan has a potential antimicrobial activity against Gram positive and Gram negative bacteria as well as fungal pathogens (Qin and Li, 2020; Cheah et al., 2019; Wei et al., 2019). Interaction and antimicrobial properties of chitosan based on the different essential environmental factors, including virulence factors, pH of medium and bacterial membrane structural protein properties and their concentration of composite materials. Bactericidal activity of chitosan is based on the type of microorganisms due to their membrane proteins. Biopolymer of chitosan possesses good anti-bacterial activity against Gram-positive bacteria of Bacillus megaterium, S. aureus, Listeria monocyogenes, Bacillus cereus, Lactobacillus plantarum, Lactobacillus bulgaricus and Lactobacillus brevis and Gram negative bacterial of E. coli, Pseudomonas fluorescens, Salmonella typhimurium, Pseudomonas aeruginosa, Vibrio cholera, Vibrio parahaemolyticus and Enterobacteraerogenes (Sahariah, 2017; Hosseinnejad and afari 2016).Antimicrobial activity of quaterized derivative chitosan has better bactericidal activity against the Gram positive bacteria of S. aureus than compared to Gram negative bacteria of E. coli reported by Alessandro (Alessandro et al., 2014). In general, the chitosan antibacterial properties derivative differs against from Gram positive and Gram positive bacteria are somewhat controversial. In some researchers reported, unmodified chitosan usually strong act against Gram negative than on Gram positive bacterial strains. Such excellent antibacterial efficiency attributed due to their cell membrane characteristic, that consist thinner cell wall of Gram negative bacteria and consequently more susceptible than the Gram positive bacteria (no et al., 2002; Silva et al., 2010).
Several researchers have been reported, the electrostatic interaction between the negatively charged bacterial membrane and positively charged chitosan is assumed as the major antibacterial mechanism (Rabea et al., 2003). The peptidoglycan layer of the bacterial membrane can play important role in providing a rigidity of structure and which can act barrier against chitosan interactions (Zheng and Zhu 2003). The thickness of E. coli cell membrane around 7-8nm while the compared to Gram positive bacteria of S. aureus is 20-80 nm thus difference can difficult to bacterial cellular lysis (Eaton et al., 2008). Another research reported, mannose functionalized chitosan has enhanced antibacterial activity of multidrug resistant pathogenic bacteria of Escherichia coli and Listeria monocytogenes (Sadaf et al., 2020).
2.4. Tissue engineering
Skin skeleton is largest organ of the human body system, which is protect from external environmental factors including mutagenic chemical substance, pollutant and pathogenic microorganisms (Kabashima et al., 2019). Currently are available several options to treatment of tissue injuries as including skin grafting techniques, which is improper and delayed healing as well as trigger negative immunogenic response. Therefore, tissue engineering immediately required promising and effective healing of naturally derived bio-materials of this area. The biopolymer based composites are potential enhancing cell growth and proliferation and, moreover, growth factor embedded biomaterials significantly improving cell differentiation and migration of skin cells (Pereira and Bártolo, 2016; Liu et al., 2018; Shpichka et al., 2019). Chitosan based developed composites or nanocomposites are suitable for tissue engineering due to their major biological properties of biodegradable, biocompatible and non-toxic.
Biopolymer of chitosan loaded with glycerol and antibacterial agents, as adding of glycerol enhancing the stability of the membrane while antibacterial agents provided long inhibition against Gram negative and Gram positive bacteria of Escherichia coli and S. aureus. The in-vitro study showed good proliferation of the fibroblast cells on the developed membrane. The results revealed that prepared membrane has potential applicable to antimicrobial dressing materials for skin burn treatment (Ma et al., 2017). Another study reported, chitosan with aloe vera-curcuminembedded membrane scaffold was investigated regeneration of skin tissue in wound healing properties. The physicochemical property of prepared membrane was appropriated and exhibit antimicrobial activity of against pathogenic microbes. Cytotoxicity of the prepared membrane was performed mouse fibroblast NIH-3T3 cells culture on the membrane, which efficiently proliferated cell growth. The chitosan, aloe vera-curcumin loaded membrane has efficiently enhancing wound healing and along with inhibition of microbial pathogens (Liu et al., 2019). Similarly, aggregated with another research report, chitosan and silk microfibers membrane scaffold was prepared to evaluated physiochemical and mechanical properties which suitable for biological applications. Scaffold was revealed no toxicity effect on L929 cells and also promoted cell proliferation. Moreover, in-vivo study suggested reduction of inflammation response, and stimulated uniform distribution of fibroblast as well as collagen deposition (Xu et al., 2015)
Conclusion
The review focused on current application of chitosan based biomaterials and it was discussed future prospects of natural derived or synthetic materials along with biopolymers combined scaffold used for various biomedical applications. Chitosan is natural biopolymer of polysaccharides have potential various biomedical applications of antimicrobial activity, drug delivery, wound healing and tissue engineering. The incorporation of chitosan based developed biomaterials potential applicable for several pharmaceutical and drug manufacturing industries, due to their excellent biological characteristic of nontoxic, biodegradable and biocompatible. In the review article concluded the chitosan based biomaterials applications are need translated from laboratory stage to further examination are required to use as commercial applicable for pharmaceutical and biomedical in the clinical applications.
Funding: The authors received no specific funding for this work.
Conflicts of Interest: None
References
- Abdou, E.S., Nagy, K.S.A., Elsabee, M.Z., 2008. Extraction and characterization of chitin and chitosan from local sources, Bioresources Technology. 99, 1359-1367
- Adeli, H., Khorasani, M. T., &Parvazinia, M., 2019. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. International Journal of Biological Macromolecules, 122, 238–254
- Alavarse, A. C., de Oliveira Silva, F. W., Colque, J. T., da Silva, V. M., Prieto, T., Venancio, E. C., Bonvent, J.-J. (2017). Tetracycline hydrochloride-loaded electrospunnanofibers mats based on PVA and chitosan for wound dressing. Materials Science and Engineering: C, 77, 271–281.
- Alessandro, F.M., Suelen, P.F., Heveline, D.M., Antonio, G.B.P., Adley, F.R., Edvani, C.M., 2014. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci.15, 20800-20832;
- Anastasia, S., Denis, B., Evgeny, A., Bezrukov, R., Sukhanov, B., Anthony, A., Vitaliy, B., Yuanyuan, Zhang., Peter, Timashev., Skin tissue regeneration for burn injury. Stem Cell Res Ther, 10, 94.
- Badawy, M. E. I., Rabea, E. I., Rogge, T. M., 2004. Synthesis and fungicidal activity of new N,O-acyl chitosan derivatives. Biomacromolecules. 5, 589–595
- Badawy, M.E.I., Rabea, E.I.., Taktak, N.E.M., 2014. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens, Carbohydrate Polymers 111, 670-682.
- Behera, S.S., Das, U., Kumar, A., Bissoyi, A., Singh, A.K., 2017. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: application in wound dressing and skin regeneration, Int. J. Biol. Macromol. 98, 329–340.
- Bordi, F., Cametti, C., Paradossi, G., 1991. Dielectric behavior of polyelectrolyte solutions: the role of proton fluctuation, J. Phys. Chem. 95 (1991) 4883-4889
- Bozzuto, G., Molinari, A., 2015. Liposomes as nanomedical devices. Int. J. Nanomedicine 10, 975–999.
- Chanda, A., Adhikari, J., Ghosh, A., Chowdhury, S. R., Thomas, S., Datta, P., Saha, P. 2018. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol, 116, 774–785.
- Cheah, W.Y., Show, P.L., Ng, I.S., Lin, G.Y., Chiu, C.Y., Chang, Y.K., 2019. Antibacterial activity of quaternized chitosan modified nanofiber membrane, Int. J. Biol. Macromol.126, 569–577.
- Chen, H., Xing, X., Tan, H., Jia, Y., Zhou, T., Chen, Y., Ling, Z., Hu, X., 2017. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing, Mater. Sci. Eng. C 70, 287–295.
- Cong, Z., Shi, Y., Wang, Y., Wang, Y., Niu, J., Chen, N., Xue, H., 2017. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol 17, 32042-1.
- Daniela, A, Liliana, M-T., Luminita, M., 2020. Citryl-imine-PEG-ylated chitosan hydrogels – Promising materials for drug delivery applications. Int. J. Biol. Macromol 62, 1323-337.
- Datta, S., Rameshbabu, A. P., Bankoti, K., Maity, P. P., Das, D., Pal, S., &Dhara, S. 2017.Oleoyl-chitosan-based nanofiber mats impregnated with amniotic membrane derived stem cells for accelerated full -thickness excisional wound healing. ACS Biomater. Sci. Eng, 3(8), 1738–1749.
- Dong, X., Yuan, Y.. Wang, L., Liu, J., Midgley, A.C., Wang, K., 2018. Construction of a bilayered vascular graft with smooth internal surface for improved hemocompatibility and endothelial cell monolayer formation, Biomaterials 181, 1-14
- Dutta , P.K., Ravikumar, M.N.V., Dutta, J., 2002. Chitin and chitosan for versatile applications. JMS Polym Rev. 42, 307
- Eaton, P., Fernandes, J.C., Pereira, E., Pintado, M.E., Malcata, F.X., 2008. Atomic forcemicroscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 108, 1128–1134.
- El-Alfy, E.A., El-Bisi, M.K., Taha, G.M., Ibrahim, H.M.,Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol 161, 1247-1260.
- Fatemeh, G., Farzaneh, A., Dariush, S., 2021. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol, 174, 278-288
- Francesca, S., Anna G.M., Giuseppe, M., Laura, B., Francesca, G., Elisabetta, M., Cosimino, M., Clara, P., 2021. Sustainable chitosan-based electrical responsive scaffolds for tissue engineering applications. SM&T 28, e00260
- Ghannam, S., Korayem, H., Farghaly, L., Hosny, S., 2018. The effect of chitosan nanosilver dressing versus mesenchymal stem cells on wound healing, Journal of African Association of Physiological, Sciences 6, 23–31
- Gomes, S., Rodrigues, G., Martins, G., Henriques, C., & Silva, J. C. 2017. Evaluation of nanofibrous scaffolds obtained from blends of chitosan, gelatin and polycaprolactone for skin tissue engineering. Int. J. Biol. Macromol, 102, 1174–1185.
- Gomes, S., Rodrigues, G., Martins, G., Roberto, M., Mafra, M., Henriques, C., Silva, J. 2015. In vitro and in vivo evaluation of electrospunnanofibers of PCL, chitosan and gelatin: A comparative study. Materials Science and Engineering: C, 46, 348–358.
- Gorochovceva, N., Makusuka, R., 2004. Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft copolymers. EurPolym J. 40, 685–91
- Hairui, S., Jiaying, Z., Mingen, X, Ling, W., 2021. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C, 123, 111963.
- Hall, L.D., Yalpani, M., 1980. Formation of branched-chain, soluble polysaccharides from chitosan. J ChemSocChemCommun, 1153–4
- Hasan, A., Waibhaw, G., Saxena, V., &Pandey, L. M. 2018. Nano-biocompositescaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modifedcellulosenanowhiskersfor bone tissue engineering applications. Int. J. Biol. Macromol, 111, 923–934.
- He, H., Lu, Y., Qi, J., Zhu, Q., Chen, Z., Wu, W., 2019. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 9 1,36–48
- He, L., Chenyu, W., Chen, L., Yanguo, Q., Zhonghan, W., Fan, Y., Zuhao, L., Jincheng, W., 2018. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 8, 7533-7547.
- He, Y., Jin, Y., Wang, X., Yao, S., Li, Y., Wu, Q., & Liu, H., 2018. An antimicrobial peptide-loaded Gelatin/Chitosan nanofibrous membrane fabricated by sequential layer-by-layer electrospinning and electrospraying techniques. Nanomaterials (Basel,Switzerland), 8(5)
- Hongli, Y., Junwen, C., Kun, Y., 2019. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol, 121, 633-642.
- Hosseinnejad, M., afari, S.M., 2016. Evaluation of different factors affecting antimicrobial properties of chitosan, Int. J. Biol. Macromol. 85, 467–475
- Inmaculada, A., Marian, M., Ruth, H., Ines, P., Beatriz. M., Niuris, A., Gemma, G., Angeles, H., 2009. Functional Characterization of Chitin and Chitosan. Current Chemical Biology. 3, 203-230
- Jayakumar, R., Prabaharan, M., Reis, R.L., M ano, J.F., 2005. Graft copolymerized chitosan-present status and applications. CarbohydrPolym. 62, 142–215.
- Jenkins, D.W., Hudson, S.M., 2001. Review of Vinyl Graft Copolymerization Featuring Recent Advances toward Controlled Radical-Based Reactions and Illustrated with Chitin/Chitosan Trunk Polymers, Chem Rev. 101, 3245–73
- Jiang, T., James, R., Kumbar, S.G., Laurencin, C.T., 2014. Chapter 5 – Chitosan as a Biomaterial: Structure, Properties, and Applications in Tissue Engineering and Drug Delivery, Natural and Synthetic Biomedical Polymers, Elsevier, Oxford, 91-113
- Johnston, M.J.W., Semple, S.C., Klimuk, S.K., Ansell, S., Maurer, N., Cullis, S. K., 2007. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim. Biophys. Acta Biomembr.1768, 1121–1127.
- Kabashima, K., Honda, T., Ginhoux, F., Egawa, G., 2019. The immunological anatomy of the skin. Nat. Rev. Immunol. 19, 19–30.
- Karakecili, A., Topuz, B., Korpayev, S., &Erdek, M. 2019. Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds Materials for Biological Applications. Mater. Sci. Eng. C, 105, 10098
- Karimi, A., Karbasi, S., Razavi, S., &Zargar, E. N. 2018. Poly (hydroxybutyrate)/chitosan aligned electrospun scaffold as a novel substrate for nerve tissue engineering. Advanced Biomedical Research, 7.
- Kaya, M., Akata, I., Baran, T., Menteş, A., 2015a. Physicochemical properties of chitin and chitosan produced from medicinal fungus (Fomitopsispinicola). Food Biophys. 10, 162–168
- Khorasani, M. T., Joorabloo, A., Moghaddam, A., Shamsi, H., &MansooriMoghadam, Z. (2018). Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol, 114, 1203–1215.
- Li, B., Wang, X., Chen, R., Huangfu, W., Xie, G., 2008. Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima, Carbohydrate Polymers 72, 287-292.
- Liu, X.C., You, L.J., Tarafder, S., Zou, L., Fang, Z.X., Chen, J.D., Lee, C.H., Zhang, Q.Q., 2019. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem. Eng. J. 359, 1111–1119.
- Liu, Y., Wang, S., & Zhang, R. 2017. Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol, 103, 1130–1137
- Ma, Y., Xin, L., Tan, H.P., Fan, M., Li, J.L., Jia, Y., Ling, Z.H., Chen, Y., Hu, X.H., 2017. Chitosan membrane dressings toughened by glycerol to load antibacterial drugs for wound healing. Mater. Sci. Eng.: C 81, 522–531
- MadhusudanaRao, K., Kumar, A., & Han, S. S. 2017. Polysaccharide based bionanocomposite hydrogels reinforced with cellulose nanocrystals: Drug release and biocompatibility analyses. Int. J. Biol. Macromol, 101, 165–171.
- Naseri-Nosar, M., Salehi, M., Farzamfar, S., &Azami, M., 2018. The single and synergistic effects of montmorillonite and curcumin-loaded chitosan microparticles incorporated onto poly(lactic acid) electrospun film on wound-healing. J BioactCompatPolym, 33(3), 239–253.
- Nessa, F., Shah, M.M., Asaduzzaman, M., Roy, S.K., Hossain, M.M., Jahan, M.S., 2010. A Process for the Preparation of Chitin and Chitosan from Prawn Shell Waste. Bangladesh J. Sci. Ind. Res. 45, 323-330.
- No, H.K., Park, N.Y., Lee, S.H., Meyers, S.P., 2002. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 74, 65–72
- Pawar, V., &Srivastava, R. 2019. Chitosan-polycaprolactoneblend sponges for management of chronic osteomyelitis: A preliminary characterization and in vitro evaluation. Int. J. Biol. Macromol, 568, Article 118553.
- Pengpeng, D., Juanjuan, C., Lichao,Y., Pingan, Z., Jinping, Z., 2021. Thymine-modified chitosan with broad-spectrum antimicrobial activities for wound healing. Carbohydr. Polym, 257, 117630.
- Pereira, R.F., Bártolo, P.J., 2016. Traditional therapies for skin wound healing. Adv. Wound Care (New Rochelle) 5, 208–229.
- Pillai, C.K.S., Paul , W., Sharma, C. P., 2009. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Scie. 34 (2009) 641–678
- Poornima, B., &Korrapati, P. S. 2017. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym, 157, 1741–1749
- Qin, Y., Li, P., 2020. Antimicrobial chitosan conjugates: current synthetic strategies and potential applications, Int. J. Mol. Sci. 21, 499
- Rabea, E.I., Badawy, M.E.-T., Stevens, C.V., Smagghe, G., Steurbaut, W., 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4, 1457–1465
- Radmansouri, M., Bahmani, E., Sarikhani, E., Rahmani, K., Sharifianjazi, F., &Irani, M. 2018. Doxorubicin hydrochloride – Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol, 116, 378–384.
- Radwan, N. H., Nasr, M., Ishak, R. A. H., Abdeltawab, N. F., &Awad, G. A. S. 2020.Chitosan-calcium phosphate composite scaffolds for control of post-operative osteomyelitis: Fabrication, characterization, and in vitro-in vivo evaluation. Carbohydr. Polym, 244, Article 116482
- Ren, Y., Zhao, X., Liang, X., Ma, P. X., &Guo, B. 2017. Injectable hydrogel based on quaternized chitosan: gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol, 105, 1079–1087
- Rha, C.K., Rodriduez- Sanchez, D., Kienzle- Sterzer, C., Colwell, R.R., Pariser, E.R., Sinskey, A.J., 1984. Novel applications of chitosan. In: Biotechnology of Marine Polysaccharide. Hemisphere Publishing, Washington, pp. 284-311
- Rijal, N. P., Adhikari, U., Khanal, S., Pai, D., Sankar, J., &Bhattarai, N. 2018. Magnesium oxide-poly(ε-caprolactone)-chitosan-based composite nanofiber for tissue engineering applications.MSEB, 228, 18–27
- Sadaf, E., Ayesha, I., Tayyaba, N., Saima, S., Muhammad, I., 2020. Mannose functionalized chitosan nanosystems for enhanced antimicrobial activity against multidrug resistant pathogens, Polym, Test. 91, 106814.
- Sahariah, H.M., 2017. Antimicrobial properties of chitosan and chitosan derivatives, in: S.-K. Kim (Ed.), Marine Glycobiology: Principles and Applications, CRC Press, 345–369.
- Sarhan, W. A., Azzazy, H. M., & El-Sherbiny, I. M., 2016. Honey/chitosan nanofiber wound dressing enriched with Allium sativum and Cleome droserifolia: Enhanced antimicrobial and wound healing activity. ACS Applied Materials & Interfaces, 8, 6379–6390
- Semnani, D., Naghashzargar, E., Hadjianfar, M., DehghanManshadi, F., Mohammadi, S., Karbasi, S., Effaty, F. 2017. Evaluation of PCL/chitosan electrospunnanofibers for liver tissue engineering. Int. J. Quantum Chem. 66(3), 149–157
- Silva, L.P., Britto, D., Seleghim, M.H.R., Assis, O.B.G., 2010. In vitro activity of watersoluble quaternary chitosan chloride salt against E coli. World J. Microbiol. Biotechnol. 26, 2089–2092
- Soubhagya, A.S., Moorthi, A., Prabaharan, M., 2020. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol, 157, 135-145
- Struszczyk, H., Wawro, D., Niekraszewicz, A., Brine, C.J., Sandford, P.A., Zikakis, J.P., 1991. Biodegradability of chitosan fibers. In: Advances in Chitin and Chitosan. Applied Science, London, 580-585.
- Suwei, J., Jian, Lv., Man, D., Yanan, L., Hualin, W., Shaotong, J., 2016. Release behavior of tetracycline hydrochloride loaded chitosan/poly(lactic acid) antimicrobial nanofibrous membranes. Materials Science and Engineering: C, 59, 86-91.
- Tonda-Turo, C., Ruini, F., Ramella, M., Boccafoschi, F., Gentile, P., Gioffredi, E., &Ciardelli, G. 2017. Non-covalently crosslinked chitosan nanofibrous mats prepared by electrospinning as substrates for soft tissue regeneration. Carbohydrate Polymers, 162, 82–92.
- Van Tran, J.-Y. Moon, Y.-C. Lee., 2019. Liposomes for delivery of antioxidants incosmeceuticals: challenges and development strategies. J. Control. Release 300 114–140
- Wei, L., Tan, W., Wang, G., Li, Q., Dong, F., Guo, Z., 2019. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts, Carbohydr. Polym. 226, 115256
- Xu, W., Wang, Z., Liu, Y., Wang, L., Jiang, Z., Li, T., Zhang, W., Liang, Y., 2018. Carboxymethyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohydr. Polym. 192, 240–250.
- Xu, Z.P., Shi, L.Y., Yang, M.Y., Zhang, H.P., Zhu, L.J., 2015. Fabrication of a novel blended membrane with chitosan and silk microfibers for wound healing: characterization, in vitro and in vivo studies. J. Mater. Chem. B 3, 3634–3642.
- Yoshifuji, A., Noishiki, Y., Wada, M., Heux, L., Kuga, S., 2006. Esterification of beta-chitin via intercalation by carboxylic anhydrides. Biomacromolecules. 7, 2878–81
- Yu, S., Zhang, X., Tan, G., Tian, L., Liu, D., Liu, Y., & Pan, W. 2017. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamercrosslinked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym, 155, 208–217.
- Zheng, L-Y., Zhu, J-F., 2003. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 54, 527–630.
- Zhou, Dong, Q., Yang, H., Liu, X., Yin, X., Tao, Y., Xu, W., 2017. Photocrosslinkedmaleilated chitosan/methacrylated poly (vinyl alcohol) bicomponentnanofibrous scaffolds for use as potential wound dressings. Carbohydrate Polymers, 168, 220-226
